How Many Electrons Can Exist in an Orbital
But what about the electron-electron repulsion. How many electrons are in 5d orbital.
How Many Electrons Are In Each Shell Including 3p Orbitals
How many electrons can exist in an orbital.
. Atomic orbital n5 l 01234 so for l1 we have Magnetic quantum number -101 Not counting spin number you add every combination allowed. From Table below we see that we can have three possible orbitals when l 1. However the electron can exist in spin up m s 12 or with spin down m s -12 configurations.
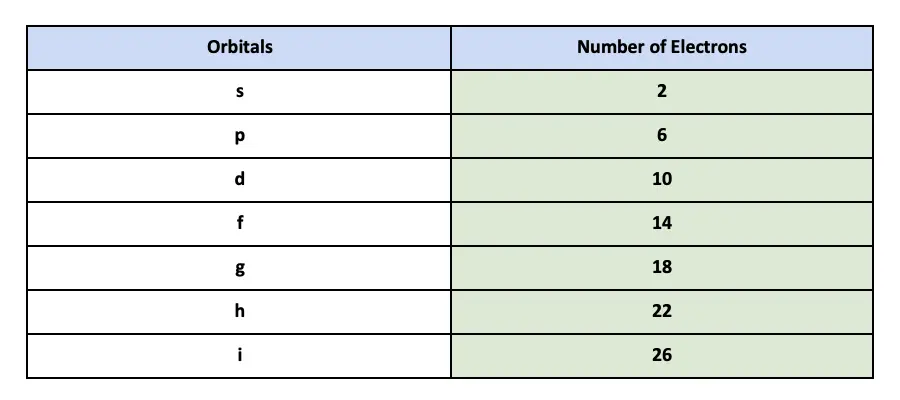
An s-orbital holds 2 electrons. Therefore the p orbital can hold 6 electrons. P1221 1 6.
There are two planar node normal to the axis of the orbital so the 5dxy orbital has yz and xz nodal planes for instance. The simple names s orbital p orbital d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ 0 1 2 and 3 respectively. 31- Each orbital can hold a maximum of 2 electrons.
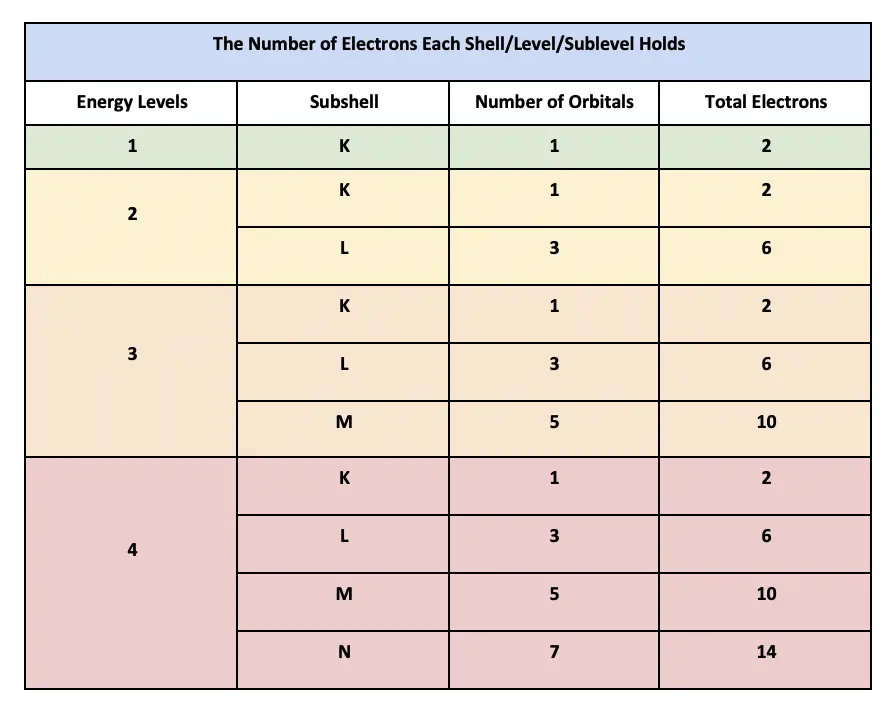
Thus to find the number of electrons possible per shell. First we look at the n1 shell the first shell. The s subshell possesses one.
How many electrons are unpaired in the orbitals of nitrogen. N1 is 1 n2 is 4 n3 is 9 n4 is 16 Notice that degeneracy is n squared. We look at the four quantum numbers for a given electron and then assign that electron to a specific orbital.
How many electrons are unpaired in the orbitals of carbon. Each orbital can contain a maximum of two electrons. Which element is represented by the electron configuration 1s22s22p2.
This orbital is spherical in shape. The subshells which include s p d and f the contain the orbitals. This means that the s orbital can contain up to two electrons the p orbital can contain up to six electrons the d orbital can contain up to 10 electrons and the f orbital can contain up to 14 electrons.
What does 5d orbitals look like. Each orbital can hold up to two electrons meaning that the 1s 2s 3s 4s and 5s can hold two electrons. I got that the nuclear charge rather the large Z-effective overcome this repulsion by pulling them together towards the nucleusOne thing more turned out in mind that it may be the attraction of the two unlike pole of the magnet developed due to opposite spin of the electrons.
Two electron of opposite spin can lie in a single orbital. For any value of n a value of l 0 places that electron in an s orbital. To calculate electron shell capability you first need to determine the number of electrons possible per shell then apply the 2n 2 formula.
The sublevels each contain a different number of orbitals. The p orbital has three sub levels with the possibility of two electrons in each suborbital. A 1 B 2 C 3 D 4 Answer.
Which orbital would the electron of a ground state hydrogen atom occupy. For example s - subshell has 1 orbital so it can hold 2 electrons p subshell View the full answer. If ℓ is the angular quantum number of subshell then maximum electrons it can hold is 22ℓ 1 Sub-shellℓMaximum electrons.
How many electrons can exist in an orbital. A 14 B 5 C 9 D 3. Orbitals are orbit paths for electrons within the sublevels of an electron shell.
Thus n1 shell can hold two electrons. 10 electrons The 3d and 4d orbitals are filled with 10 electrons each and the 5d orbital has 10 electrons giving a total of 30. Each such orbital can be occupied by a maximum of two electrons each with its own projection of spin.
An orbital can hold a pair of electrons each possessing a different spin. Answer 1 of 2. The 3d 4d etc can each hold ten electrons because they each have five orbitals and each orbital can hold two electrons 5210.
How many electrons can exist in an orbital. The lowest energy orbital in the quantum-mechanical model is the. That is 25 orbitals in level n5.
As a consequence of Paulis exclusion principle each orbital can have a maximum of two electrons. A s subshell. Students should confuse orbitals with the subshells which are different.
So there are a total of five d orbitals in a particular value of principle quantum number n So 5 d orbitals will accommodate a total of 5210 electrons. The 2p3p 4p and 5p can each hold six electrons because they have three orbitals. The subshell that has three orbitals and can hold up to six electrons is the.
D electrons are very important in oxidation-reduction chemistry. The 5dz 2 orbital is. S0220 1 2.
How Many Electrons Can The Third Energy Level Hold At Level
How Many Electrons Are In Each Shell Including 3p Orbitals
Electron Arrangement In Atoms Elements And The Periodic Table

How Many Electrons Can The Fourth Energy Level Hold At Level
Comments
Post a Comment